RSA (Ranger Success Academics)
Written by Quantum Study Club on 10.47RSA (Ranger Success Academics)
-"Mengantarkan Anggota Power Ranger Meraih IP 4"-
Dosen: Prof. Arip Nurahman PhD, D.Ed, D.Sc. C. Alm. (Calon Almarhum )
Faculty of Sciences and Mathematics, Indonesia University of Education
and
Follower Open Course Ware at Massachusetts Institute of Technology
Cambridge, USA
Department of Physics
http://web.mit.edu/physics/
http://ocw.mit.edu/OcwWeb/Physics/index.htm
&
Aeronautics and Astronautics Engineering
http://web.mit.edu/aeroastro/www/
http://ocw.mit.edu/OcwWeb/Aeronautics-and-Astronautics/index.htm


Judul Kuliah: Fisika Quantum (Sejarah dan Implikasinya)
Mekanika kuantum adalah cabang dasar fisika yang menggantikan mekanika klasik pada tataran atom dan subatom. Ilmu ini memberikan kerangka matematika untuk berbagai cabang fisika dan kimia, termasuk fisika atom, fisika molekular, kimia komputasi, kimia kuantum, fisika partikel, dan fisika nuklir. Mekanika kuantum adalah bagian dari teori medan kuantum dan fisika kuantum umumnya, yang, bersama relativitas umum, merupakan salah satu pilar fisika modern. Dasar dari mekanika kuantum adalah bahwa energi itu tidak kontinyu, tapi diskrit -- berupa 'paket' atau 'kuanta'. Konsep ini revolusioner -- bertentangan dengan fisika klasik yang berasumsi bahwa energi itu berkesinambungan.
Sejarah
Pada tahun 1900, Max Planck memperkenalkan ide bahwa energi dapat dibagi-bagi menjadi beberapa paket atau kuanta. Ide ini secara khusus digunakan untuk menjelaskan sebaran intensitas radiasi yang dipancarkan oleh benda hitam. Pada tahun 1905, Albert Einstein menjelaskan efek fotoelektrik dengan menyimpulkan bahwa energi cahaya datang dalam bentuk kuanta yang disebut foton. Pada tahun 1913, Niels Bohr menjelaskan garis spektrum dari atom hidrogen, lagi dengan menggunakan kuantisasi. Pada tahun 1924, Louis de Broglie memberikan teorinya tentang gelombang benda.
Teori-teori di atas, meskipun sukses, tetapi sangat fenomenologikal: tidak ada penjelasan jelas untuk kuantisasi. Mereka dikenal sebagai teori kuantum lama.
Frase "Fisika kuantum" pertama kali digunakan oleh Johnston dalam tulisannya Planck's Universe in Light of Modern Physics (Alam Planck dalam cahaya Fisika Modern).
Mekanika kuantum modern lahir pada tahun 1925, ketika Werner Karl Heisenberg mengembangkan mekanika matriks dan Erwin Schrödinger menemukan mekanika gelombang dan persamaan Schrödinger. Schrödinger beberapa kali menunjukkan bahwa kedua pendekatan tersebut sama.
Heisenberg merumuskan prinsip ketidakpastiannya pada tahun 1927, dan interpretasi Kopenhagen terbentuk dalam waktu yang hampir bersamaan. Pada 1927, Paul Dirac menggabungkan mekanika kuantum dengan relativitas khusus. Dia juga membuka penggunaan teori operator, termasuk notasi bra-ket yang berpengaruh. Pada tahun 1932, Neumann Janos merumuskan dasar matematika yang kuat untuk mekanika kuantum sebagai teori operator.
Bidang kimia kuantum dibuka oleh Walter Heitler dan Fritz London, yang mempublikasikan penelitian ikatan kovalen dari molekul hidrogen pada tahun 1927. Kimia kuantum beberapa kali dikembangkan oleh pekerja dalam jumlah besar, termasuk kimiawan Amerika Linus Pauling.
Berawal pada 1927, percobaan dimulai untuk menggunakan mekanika kuantum ke dalam bidang di luar partikel satuan, yang menghasilkan teori medan kuantum. Pekerja awal dalam bidang ini termasuk Dirac, Wolfgang Pauli, Victor Weisskopf dan Pascaul Jordan. Bidang riset area ini dikembangkan dalam formulasi elektrodinamika kuantum oleh Richard Feynman, Freeman Dyson, Julian Schwinger, dan Tomonaga Shin'ichirō pada tahun 1940-an. Elektrodinamika kuantum adalah teori kuantum elektron, positron, dan Medan elektromagnetik, dan berlaku sebagai contoh untuk teori kuantum berikutnya.
Interpretasi banyak dunia diformulasikan oleh Hugh Everett pada tahun 1956.
Teori Kromodinamika kuantum diformulasikan pada awal 1960an. Teori yang kita kenal sekarang ini diformulasikan oleh Polizter, Gross and Wilzcek pada tahun 1975. Pengembangan awal oleh Schwinger, Peter Higgs, Goldstone dan lain-lain. Sheldon Lee Glashow, Steven Weinberg dan Abdus Salam menunjukan secara independen bagaimana gaya nuklir lemah dan elektrodinamika kuantum dapat digabungkan menjadi satu gaya lemah elektro.
Eksperimen penemuan
- Eksperimen celah-ganda Thomas Young membuktikan sifat gelombang dari cahaya. (sekitar 1805)
- Henri Becquerel menemukan radioaktivitas (1896)
- Joseph John Thomson - eksperimen tabung sinar kathoda (menemukan elektron dan muatan negatifnya) (1897)
- Penelitian radiasi benda hitam antara 1850 dan 1900, yang tidak dapat dijelaskan tanpa konsep kuantum.
- Robert Millikan - eksperimen tetesan oli, membuktikan bahwa muatan listrik terjadi dalam kuanta (seluruh unit), (1909)
- Ernest Rutherford - eksperimen lembaran emas menggagalkan model puding plum atom yang menyarankan bahwa muatan positif dan masa atom tersebar dengan rata. (1911)
- Otto Stern dan Walter Gerlach melakukan eksperimen Stern-Gerlach, yang menunjukkan sifat kuantisasi partikel spin (1920)
- Clyde L. Cowan dan Frederick Reines meyakinkan keberadaan neutrino dalam eksperimen neutrino (1955)
Bukti dari mekanika kuantum
Mekanika kuantum sangat berguna untuk menjelaskan apa yang terjadi di mikroskopic level, misalnya elektron di dalam atom. Atom biasanya digambarkan sebagai sebuah sistem di mana elektron (yang bermuatan listrik negatif) beredar seputar nukleus (yang bermuatan listrik positif). Mengapa elektron tidak tertarik menuju nukleus dan melepaskan energinya? Mengapa ada diskrit energi level? Menurut mekanika kuantum, ketika sebuah elektron berpindah dari energi level yang lebih tinggi (misalnya n=2) ke energi level yang lebih rendah (misalnya n=1), energi berupa sebuah cahaya partikel, foton, dilepaskan:
di mana
Dalam spektrometer masa, telah dibuktikan bahwa garis-garis spektrum dari atom yang di-ionisasi tidak kontinyu; hanya pada frekuensi/panjang gelombang tertentu garis-garis spektrum dapat dilihat. Ini adalah salah satu bukti dari teori mekanika kuantum.
Quantum
In physics, a quantum (plural: quanta) is an indivisible entity of a quantity that has the same units as the Planck constant and is related to both energy and momentum of elementary particles of matter (called fermions) and of photons and other bosons. The word comes from the Latin "quantus," for "how much." Behind this, one finds the fundamental notion that a physical property may be "quantized", referred to as "quantization". This means that the magnitude can take on only certain discrete numerical values, rather than any value, at least within a range. There is a related term of quantum number.
A photon is often referred to as a "light quantum." The energy of an electron bound to an atom (at rest) is said to be quantized, which results in the stability of atoms, and of matter in general. But these terms can be a little misleading, because what is quantized is this Planck's constant quantity whose units can be viewed as either energy multiplied by time or momentum multiplied by distance.
Usually referred to as quantum "mechanics," it is regarded by virtually every professional physicist as the most fundamental framework we have for understanding and describing nature at the infinitesimal level, for the very practical reason that it works. It is "in the nature of things", not a more or less arbitrary human preference.
Contents |
Development of quantum theory
Quantum theory, the branch of physics which is based on quantization, began in 1900 when Max Planck published his theory explaining the emission spectrum of black bodies. In that paper Planck used the Natural system of units he invented the previous year. The consequences of the differences between classical and quantum mechanics quickly became obvious. But it was not until 1926, by the work of Werner Heisenberg, Erwin Schrödinger, and others, that quantum mechanics became correctly formulated and understood mathematically. Despite tremendous experimental success, the philosophical interpretations of quantum theory are still widely debated.
Planck was reluctant to accept the new idea of quantization, as were many others. But, with no acceptable alternative, he continued to work with the idea, and found his efforts were well received. Eighteen years later, when he accepted the Nobel Prize in Physics for his contributions, he called it "a few weeks of the most strenuous work" of his life. During those few weeks, he even had to discard much of his own theoretical work from the preceding years. Quantization turned out to be the only way to describe the new and detailed experiments which were just then being performed. He did this practically overnight, openly reporting his change of mind to his scientific colleagues, in the October, November, and December meetings of the German Physical Society, in Berlin, where the black body work was being intensely discussed. In this way, careful experimentalists (including Friedrich Paschen, O.R. Lummer, Ernst Pringsheim, Heinrich Rubens, and F. Kurlbaum), and a reluctant theorist, ushered in a momentous scientific revolution.
The quantum black-body radiation formula
When a body is heated, it emits radiant heat, a form of electromagnetic radiation in the infrared region of the EM spectrum. All of this was well understood at the time, and of considerable practical importance. When the body becomes red-hot, the red wavelength parts start to become visible. This had been studied over the previous years, as the instruments were being developed. However, most of the heat radiation remains infrared, until the body becomes as hot as the surface of the Sun (about 6000 K, where most of the light is green in color). This was not achievable in the laboratory at that time. What is more, measuring specific infrared wavelengths was only then becoming feasible, due to newly developed experimental techniques. Until then, most of the electromagnetic spectrum was not measurable, and therefore blackbody emission had not been mapped out in detail.
The quantum black-body radiation formula, being the very first piece of quantum mechanics, appeared Sunday evening October 7, 1900, in a so-called back-of-the-envelope calculation by Planck. It was based on a report by Rubens (visiting with his wife) of the very latest experimental findings in the infrared. Later that evening, Planck sent the formula on a postcard, which Rubens received the following morning. A couple of days later, he informed Planck that it worked perfectly. At first, it was just a fit to the data; only later did it turn out to enforce quantization.
This second step was only possible due to a certain amount of luck (or skill, even though Planck himself called it "a fortuitous guess at an interpolation formula"). It was during the course of polishing the mathematics of his formula that Planck stumbled upon the beginnings of Quantum Theory. Briefly stated, he had two mathematical expressions:
- (i) from the previous work on the red parts of the spectrum, he had x;
- (ii) now, from the new infrared data, he got x².
Combining these as x(a+x), he still has x, approximately, when x is much smaller than a (the red end of the spectrum); but now also x² (again approximately) when x is much larger than a (in the infrared). The formula for the energy E, in a single mode of radiation at frequency λ, and temperature T, can be written
This is (essentially) what is being compared with the experimental measurements. There are two parameters to determine from the data, written in the present form by the symbols used today: h is the new Planck's constant, and k is Boltzmann's constant. Both have now become fundamental in physics, but that was by no means the case at the time. The "elementary quantum of energy" is hλ. But such a unit does not normally exist, and is not required for quantization.
Beyond electromagnetic radiation
While quantization was first discovered in electromagnetic radiation, it describes a fundamental aspect of energy not just restricted to photons.[1]
The birthday of quantum mechanics
From the experiments, Planck deduced the numerical values of h and k. Thus he could report, in the German Physical Society meeting on December 14, 1900, where quantization (of energy) was revealed for the first time, values of the Avogadro-Loschmidt number, the number of real molecules in a mole, and the unit of electrical charge, which were more accurate than those known until then. This event has been referred to as "the birth of quantum mechanics".
See also
- Quantum mechanics
- Quantum state
- Quantum number
- Quantum Leap (TV series)
- Quantum cryptography
- Quantum electronics
- Quantum computer
- Quantum entanglement
- Quantum coherence
- Quantum immortality
- Quantum lithography
- Quantum metrology
- Quantum sensor
- Quantum dot
- Magnetic flux quantum
- Quantum cellular automata
- Quantization
- Subatomic particle
- Elementary particle
- Photon polarization
References
- J. Mehra and H. Rechenberg, The Historical Development of Quantum Theory, Vol.1, Part 1, Springer-Verlag New York Inc., New York 1982.
- Lucretius, "On the Nature of the Universe", transl. from the Latin by R.E. Latham, Penguin Books Ltd., Harmondsworth 1951. There are, of course, many translations, and the translation's title varies. Some put emphasis on how things work, others on what things are found in nature.
- M. Planck, A Survey of Physical Theory, transl. by R. Jones and D.H. Williams, Methuen & Co., Ltd., London 1925 (Dover editions 1960 and 1993) including the Nobel lecture.
Notes
- ^ Real-World Quantum Effects Demonstrated February 11, 2005
History of quantum mechanics

The history of quantum mechanics as this interlaces with history of quantum chemistry began essentially with the 1838 discovery of cathode rays by Michael Faraday, during the 1859-1860 winter statement of the black body radiation problem by Gustav Kirchhoff, the 1877 suggestion by Ludwig Boltzmann that the energy states of a physical system could be discrete, and the 1900 quantum hypothesis by Max Planck that any energy radiating atomic system can theoretically be divided into a number of discrete ‘energy elements’ ε (epsilon) such that each of these energy elements is proportional to the frequency ν with which they each individually radiate energy, as defined by the following formula:
where h is a numerical value called Planck’s constant. Then, in 1905, to explain the photoelectric effect (1839), i.e. that shining light on certain materials can function to eject electrons from the material, Albert Einstein postulated, as based on Planck’s quantum hypothesis, that light itself consists of individual quantum particles, which later came to be called photons (1926). The phrase "quantum mechanics" was first used in Max Born's 1924 paper "Zur Quantenmechanik". In the years to follow, this theoretical basis slowly began to be applied to chemical structure, reactivity, and bonding.
Contents |
Overview
In short, in 1900, German physicist Max Planck introduced the idea that energy is quantized, in order to derive a formula for the observed frequency dependence of the energy emitted by a black body. In 1905, Einstein explained the photoelectric effect by postulating that light, or more specifically all electromagnetic radiation, can be divided into a finite number of "energy quanta" that are localized points in space. From the introduction section of his March 1905 quantum paper, “On a heuristic viewpoint concerning the emission and transformation of light”, Einstein states:
| “ | According to the assumption to be contemplated here, when a light ray is spreading from a point, the energy is not distributed continuously over ever-increasing spaces, but consists of a finite number of energy quanta that are localized in points in space, move without dividing, and can be absorbed or generated only as a whole. | ” |
This statement has been called the most revolutionary sentence written by a physicist of the twentieth century.[1] These energy quanta later came to be called "photons", a term introduced by Gilbert N. Lewis in 1926. The idea that each photon had to consist of energy in terms of quanta was a remarkable achievement as it effectively removed the possibility of black body radiation attaining infinite energy if it were to be explained in terms of wave forms only. In 1913, Bohr explained the spectral lines of the hydrogen atom, again by using quantization, in his paper of July 1913 On the Constitution of Atoms and Molecules.
These theories, though successful, were strictly phenomenological: during this time, there was no rigorous justification for quantization aside, perhaps, for Henri Poincaré's discussion of Planck's theory in his 1912 paper Sur la théorie des quanta.[2][3] They are collectively known as the old quantum theory.
The phrase "quantum physics" was first used in Johnston's Planck's Universe in Light of Modern Physics (1931).
In 1924, the French physicist Louis de Broglie put forward his theory of matter waves by stating that particles can exhibit wave characteristics and vice versa. This theory was for a single particle and derived from special relativity theory. Building on de Broglie's approach, modern quantum mechanics was born in 1925, when the German physicists Werner Heisenberg and Max Born developed matrix mechanics and the Austrian physicist Erwin Schrödinger invented wave mechanics and the non-relativistic Schrödinger equation as an approximation to the generalised case of de Broglie's theory [4]. Schrödinger subsequently showed that the two approaches were equivalent.
Heisenberg formulated his uncertainty principle in 1927, and the Copenhagen interpretation started to take shape at about the same time. Starting around 1927, Paul Dirac began the process of unifying quantum mechanics with special relativity by proposing the Dirac equation for the electron. The Dirac equation achieves the relativistic description of the wavefunction of an electron that Schrödinger failed to obtain. It predicts electron spin and led Dirac to predict the existence of the positron. He also pioneered the use of operator theory, including the influential bra-ket notation, as described in his famous 1930 textbook. During the same period, Hungarian polymath John von Neumann formulated the rigorous mathematical basis for quantum mechanics as the theory of linear operators on Hilbert spaces, as described in his likewise famous 1932 textbook. These, like many other works from the founding period still stand, and remain widely used.
The field of quantum chemistry was pioneered by physicists Walter Heitler and Fritz London, who published a study of the covalent bond of the hydrogen molecule in 1927. Quantum chemistry was subsequently developed by a large number of workers, including the American theoretical chemist Linus Pauling at Cal Tech, and John C. Slater into various theories such as Molecular Orbital Theory or Valence Theory.
Beginning in 1927, attempts were made to apply quantum mechanics to fields rather than single particles, resulting in what are known as quantum field theories. Early workers in this area included P.A.M. Dirac, W. Pauli, V. Weisskopf, and P. Jordan. This area of research culminated in the formulation of quantum electrodynamics by R.P. Feynman, F. Dyson, J. Schwinger, and S.I. Tomonaga during the 1940s. Quantum electrodynamics is a quantum theory of electrons, positrons, and the electromagnetic field, and served as a role model for subsequent quantum field theories. The theory of quantum chromodynamics was formulated beginning in the early 1960s. The theory as we know it today was formulated by Politzer, Gross and Wilczek in 1975. Building on pioneering work by Schwinger, Higgs and Goldstone, the physicists Glashow, Weinberg and Salam independently showed how the weak nuclear force and quantum electrodynamics could be merged into a single electroweak force, for which they received the 1979 Nobel Prize in Physics.
Timeline
The following timeline shows the key steps and contributors in the precursory development of quantum mechanics and quantum chemistry:
| Date | Person | Contribution |
| 1771 | Luigi Galvani | Noted that the muscles of dead frogs twitched when struck by a spark, to which he referred to as “animal electricity.” |
| 1800 | Alessandro Volta | Invented the voltaic pile, or "battery", specifically to disprove Galvani's animal electricity theory (1771). |
| 1803 | Thomas Young | Double-slit experiment supports the wave theory of light and demonstrates the effect of interference. |
| 1838 | Michael Faraday | Using Volta's battery (1800), he discovered “cathode rays” when, during an experiment, he passed current through a rarefied air filled glass tube and noticed a strange light arc starting at the anode (positive electrode) and ending at the cathode (negative electrode). |
| 1852 | Edward Frankland | Initiated the theory of valency by proposing that each element has a specific “combining power”, e.g. some elements such as nitrogen tend to combine with three other elements (e.g. NO3) while others may tend to combine with five (e.g. PO5), and that each element strives to fulfill its combining power (valency) quota so as to satisfy their affinities. |
| 1859 | Gustav Kirchhoff | Stated the "black body problem", i.e. how does the intensity of the electromagnetic radiation emitted by a black body depend on the frequency of the radiation and the temperature of the body? |
| 1877 | Ludwig Boltzmann | Suggested that the energy states of a physical system could be discrete. |
| 1879 | William Crookes | Showed that cathode rays (1838), unlike light rays, can be bent in a magnetic field. |
| 1885 | Johann Balmer | Discovered that the four visible lines of the hydrogen spectrum could be assigned integers in a series |
| 1888 | Johannes Rydberg | Modified Balmer formula to include the other series of lines to produce Rydberg formula |
| 1891 | Alfred Werner | Proposed a theory of affinity and valence in which affinity is an attractive force issuing from the center of the atom which acts uniformly from there towards all parts of the spherical surface of the central atom. |
| 1892 | Heinrich Hertz | Showed that cathode rays (1838) could pass through thin sheets of gold foil and produce appreciable luminosity on glass behind them. |
| 1896 | Henri Becquerel | Discovered “radioactivity” a process in which, due to nuclear disintegration, certain elements or isotopes spontaneously emit one of three types of energetic entities: alpha particles (positive charge), beta particles (negative charge), and gamma particles (neutral charge). |
| 1897 | J. J. Thomson | Showed that cathode rays (1838) bend under the influence of both an electric field and a magnetic field and to explain this he suggested that cathode rays are negatively charged subatomic electrical particles or “corpuscles” (electrons), stripped from the atom; and in 1904 proposed the “plum pudding model" in which atoms have a positively charged amorphous mass (pudding) as a body embedded with negatively charged electrons (raisins) scattered throughout in the form of non-random rotating rings. |
| 1900 | Max Planck | To explain black body radiation (1862), he suggested that electromagnetic energy could only be emitted in quantized form, i.e. the energy could only be a multiple of an elementary unit E = hν, where h is Planck's constant and ν is the frequency of the radiation. |
| 1902 | Gilbert N. Lewis | To explain the octet rule (1893), he developed the “cubical atom” theory in which electrons in the form of dots were positioned at the corner of a cube and suggested that single, double, or triple “bonds” result when two atoms are held together by multiple pairs of electrons (one pair for each bond) located between the two atoms (1916). |
| 1904 | Richard Abegg | Noted the pattern that the numerical difference between the maximum positive valence, such as +6 for H2SO4, and the maximum negative valence, such as -2 for H2S, of an element tends to be eight (Abegg's rule). |
| 1905 | Albert Einstein | To explain the photoelectric effect (1839), i.e. that shining light on certain materials can function to eject electrons from the material, he postulated, as based on Planck’s quantum hypothesis (1900), that light itself consists of individual quantum particles (photons). |
| 1907 | Ernest Rutherford | To test the plum pudding model (1904), he fired, positively-charged, alpha particles at gold foil and noticed that some bounced back thus showing that atoms have a small-sized positively charged atomic nucleus at its center. |
| 1912 | Henri Poincaré | Published an influential mathematical argument in support of the essential nature of energy quanta.[2][3] |
| 1913 | Niels Bohr | To explain the Rydberg formula (1888), which correctly modeled the light emission spectra of atomic hydrogen, Bohr hypothesized that negatively charged electrons revolve around a positively charged nucleus at certain fixed “quantum” distances and that each of these “spherical orbits” has a specific energy associated with it such that electron movements between orbits requires “quantum” emissions or absorptions of energy. |
| 1916 | Arnold Sommerfeld | To account for the Zeeman effect (1896), i.e. that atomic absorption or emission spectral lines change when the light is first shinned through a magnetic field, he suggesting that there might be “elliptical orbits” in atoms in addition to spherical orbits. |
| 1919 | Irving Langmuir | Building on the work of Lewis (1916), he coined the term "covalence" and postulated that coordinate covalent bonds occur when the electrons of a pair come from the same atom. |
| 1922 | Stern & Gerlach | Stern-Gerlach experiment detects discrete values of angular momentum for atoms in the ground state passing through an inhomogeneous magnetic field leading to the discovery of the spin of the electron. |
| 1923 | Louis De Broglie | Postulated that electrons in motion are associated with waves the lengths of which are given by Planck’s constant h divided by the momentum of the mv = p of the electron: λ = h / mv = h / p. |
| 1924 | Satyendra Nath Bose | His work on quantum mechanics provides the foundation for Bose-Einstein statistics, the theory of the Bose-Einstein condensate, and the discovery of the boson. |
| 1925 | Friedrich Hund | Outlined the “rule of maximum multiplicity” which states that when electrons are added successively to an atom as many levels or orbits are singly occupied as possible before any pairing of electrons with opposite spin occurs and made the distinction that the inner electrons in molecules remained in atomic orbitals and only the valence electrons needed to be in molecular orbitals involving both nuclei. |
| 1925 | Werner Heisenberg | Developed the matrix mechanics formulation of QM |
| 1925 | Wolfgang Pauli | Outlined the “Pauli exclusion principle” which states that no two identical fermions may occupy the same quantum state simultaneously. |
| 1926 | Erwin Schrödinger | Used De Broglie’s electron wave postulate (1924) to develop a “wave equation” that represents mathematically the distribution of a charge of an electron distributed through space, being spherically symmetric or prominent in certain directions, i.e. directed valence bonds, which gave the correct values for spectral lines of the hydrogen atom. |
| 1927 | Walter Heitler | Used Schrödinger’s wave equation (1926) to show how two hydrogen atom wavefunctions join together, with plus, minus, and exchange terms, to form a covalent bond. |
| 1927 | Robert Mulliken | In 1927 Mulliken worked, in coordination with Hund, to develop a molecular orbital theory where electrons are assigned to states that extend over an entire molecule and in 1932 introduced many new molecular orbital terminologies, such as σ bond, π bond, and δ bond. |
| 1928 | Linus Pauling | Outlined the nature of the chemical bond in which he used Heitler’s quantum mechanical covalent bond model (1927) to outline the quantum mechanical basis for all types of molecular structure and bonding and suggested that different types of bonds in molecules can become equalized by rapid shifting of electrons, a process called “resonance” (1931), such that resonance hybrids contain contributions from the different possible electronic configurations. |
| 1929 | John Lennard-Jones | Introduced the linear combination of atomic orbitals approximation for the calculation of molecular orbitals. |
| 1932 | Werner Heisenberg | Applied perturbation theory to the two-electron problem and showed how resonance arising from electron exchange could explain exchange forces. |
| 1938 | Charles Coulson | Made the first accurate calculation of a molecular orbital wavefunction with the hydrogen molecule. |
| 1951 | Clemens C. J. Roothaan and George G. Hall | Derived the Roothaan-Hall equations, putting rigorous molecular orbital methods on a firm basis. |
| 1979 | Abdus Salam, Sheldon Lee Glashow and Steven Weinberg | Showed how the weak nuclear force and quantum electrodynamics could be merged into a single electroweak force, for which they received the 1979 Nobel Prize in Physics. |
Founding experiments
- Thomas Young's double-slit experiment demonstrating the wave nature of light (c1805)
- Henri Becquerel discovers radioactivity (1896)
- J. J. Thomson's cathode ray tube experiments (discovers the electron and its negative charge) (1897)
- The study of black body radiation between 1850 and 1900, which could not be explained without quantum concepts.
- The photoelectric effect: Einstein explained this in 1905 (and later received a Nobel prize for it) using the concept of photons, particles of light with quantized energy
- Robert Millikan's oil-drop experiment, which showed that electric charge occurs as quanta (whole units), (1909)
- Ernest Rutherford's gold foil experiment disproved the plum pudding model of the atom which suggested that the mass and positive charge of the atom are almost uniformly distributed. (1911)
- Otto Stern and Walther Gerlach conduct the Stern-Gerlach experiment, which demonstrates the quantized nature of particle spin (1920)
- Clinton Davisson and Lester Germer demonstrate the wave nature of the electron[5] in the Electron diffraction experiment (1927)
- Clyde L. Cowan and Frederick Reines confirm the existence of the neutrino in the neutrino experiment (1955)
- Claus Jönsson`s double-slit experiment with electrons (1961)
- The Quantum Hall effect, discovered in 1980 by Klaus von Klitzing. The quantized version of the Hall effect has allowed for the definition of a new practical standard for electrical resistance and for an extremely precise independent determination of the fine structure constant.
- The experimental verification of quantum entanglement by Alain Aspect in 1982.
References
- ^ Folsing, Albrecht (1997). Albert Einstein: A Biography. trans. Ewald Osers, Viking.
- ^ a b McCormmach, Russell (Spring, 1967), "Henri Poincaré and the Quantum Theory", Isis 58(1): 37-55
- ^ a b Irons, F. E. (August, 2001), "Poincaré's 1911–12 proof of quantum discontinuity interpreted as applying to atoms", American Journal of Physics 69(8): 879-884
- ^ Hanle, P.A. (December 1977), "Erwin Schrodinger's Reaction to Louis de Broglie's Thesis on the Quantum Theory.", Isis 68(4): 606-609
- ^ The Davisson-Germer experiment, which demonstrates the wave nature of the electron
See also
External links
- A History of Quantum Mechanics
- A Brief History of Quantum Mechanics
- Homepage of the Quantum History Project

 adalah energi (
adalah energi ( adalah tetapan Planck,
adalah tetapan Planck,  (
(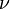 adalah frekuensi dari cahaya (
adalah frekuensi dari cahaya (
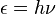
 | Posted in »
| Posted in »



















0 comments: Responses to “ RSA (Ranger Success Academics) ”